Dioxygen difluoride is a
compound with the
formula O2F2. It exists as an orange solid that melts into a red liquid at −163 °C.
[1] It is a strong oxidant and decomposes into
OF2 and oxygen even at −160 °C (4% per day) - it thus can not exist at room temperature.
[2]
Dioxygen difluoride is one of the most unstable and explosive chemicals ever synthesized. It reacts explosively with nearly every chemical it encounters, even ordinary ice, leading to its nickname, FOOF, a play on its chemical formula. Another nickname, coined by chemist Derek Lowe, is Satan's
Kimchi.
[3]
Preparation
Dioxygen difluoride can be obtained by subjecting a 1:1 mixture of gaseous fluorine and oxygen at low pressure (7–17
mmHg is optimal) to an electric discharge of 25–30
mA at 2.1–2.4
kV. This is basically the reaction used for the first synthesis by
Otto Ruff in 1933.
[4] Another synthesis involves mixing O
2and F
2 in a
stainless steel vessel cooled to −196 °C, followed by exposing the elements to
3 MeVbremsstrahlung for several hours. A third method requires heating a mix of Fluorine and Oxygen to
700 °C (1,292 °F), and then rapidly cooling it using
liquid oxygen.
[5] Fluorine is extremely dangerous and unstable at these temperatures and this synthesis should not be attempted.
[3]
Structure and electronic description
In
O2F2, oxygen is assigned the unusual
oxidation state of +1. In most of its other compounds, oxygen has an oxidation state of −2.
The structure of dioxygen difluoride resembles that of
hydrogen peroxide,
H2O2, in its large
dihedral angle, which approaches 90°. This geometry conforms with the predictions of
VSEPR theory. The O−O bond length is within 2 pm of the 120.7
pm distance for the O=O double bond in
dioxygen, O
2.
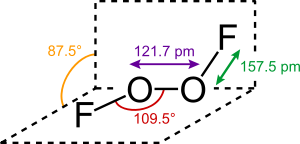
The bonding within dioxygen difluoride has been the subject of considerable speculation over the years, particularly because of the very short O–O distance and the long O–F distances. Bridgeman has proposed a scheme which essentially has an O–O
triple bond and an O–F single bond that is destabilised and lengthened by repulsion between the
lone pairs on the fluorine atoms and the
π-orbitalsof the O–O bond.
[6] Repulsion involving the fluorine lone pairs is also responsible for the long and weak
covalent bonding in the fluorine molecule. The
19F NMR chemical shift of dioxygen difluoride is 865 ppm, which is by far the highest chemical shift recorded for a fluorine nucleus, thus underlining the extraordinary electronic properties of this compound.
Reactivity
The overarching property of this unstable compound is its
oxidizing power, despite the fact that all reactions must be conducted near −100 °C.
[7] A.G. Streng conducted a series of experiments with the substance to test its reactions. Streng's experiments resulted in a series of fires and explosions. Some of the compounds that produced violent reactions with FOOF include
ethyl alcohol,
methane,
ammonia, and even with
water ice.
[7]Despite Streng's willingness to react extremely dangerous compounds, he was unable to perform some of the experiments due to their extreme explosiveness. For example, four molecules of dioxygen difluoride reacting with one molecule of
hydrogen sulfide produces 433 kilocalories of energy, which commentator Derek Lowe calls "the kind of every-man-for-himself
exotherm that you want to avoid at all cost". Lowe continues, "A. G. Streng, folks, absolutely takes the corrosive exploding cake, and I have to tip my asbestos-lined titanium hat to him."
[3]- 2 O2F2 + 2 PF5 → 2 [O2]+[PF6]− + F2
Applications
Due to its extreme instability and the low temperatures required for it to exist, there are no practical applications. In his experiments, Streng succesfully stored it in solid form at
90 K (−183.2 °C).
Los Alamos National Laboratory has reportedly used it to convert uranium and plutonium oxides into the corresponding hexafluorides
[9], but otherwise, the safety problems have prevented this chemical from seeing any use. In 2010, a company called Hangzhou Sage Chemical Company claimed to offer the chemical for sale in kilogram quantities. It is highly doubtful that they actually have the chemical, due to the problems in both storing and shipping it safely.
[3]
See Also
References
- ^ Kirshenbaum, A. D.; Grosse, A. V. (1959). "Ozone Fluoride or Trioxygen Difluoride, O3F2". Journal of the American Chemical Society 81 (6): 1277.doi:10.1021/ja01515a003.
- ^ a b Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. Academic Press. ISBN 0-12-352651-5.
- ^ a b c d Derek Lowe (February 23, 2010), "Things I Won't Work With: Dioxygen Difluoride", In the Pipeline, retrieved April 9, 2013
- ^ Ruff, O.; Mensel, W. (1933). "Neue Sauerstofffluoride: O2F2 und OF". Zeitschrift für anorganische und allgemeine Chemie 211 (1–2): 204–208.doi:10.1002/zaac.19332110122.
- ^ Thomas Mills. Direct synthesis of liquid-phase dioxygen difluoride. Retrieved April 9, 2013.(subscription required)
- ^ Bridgeman, A. J.; Rothery, J. (1999). "Bonding in mixed halogen and hydrogen peroxides". Journal of the Chemical Society, Dalton Transactions 1999 (22): 4077–4082. doi:10.1039/a904968a.
- ^ a b Streng, A. G. (1963). "The Chemical Properties of Dioxygen Difluoride". Journal of the American Chemical Society 85 (10): 1380–1385. doi:10.1021/ja00893a004.
- ^ Solomon, I. J.; et al. (1964). "New Dioxygenyl Compounds". Inorganic Chemistry 3 (3): 457. doi:10.1021/ic50013a036.
- ^ Atwood, D. A. (2006). "Fluorine: Inorganic Chemistry". Encyclopedia of Inorganic Chemistry. John Wiley & Sons. doi:10.1002/0470862106.ia076.
External links